UNIVERSAL access
to medicines
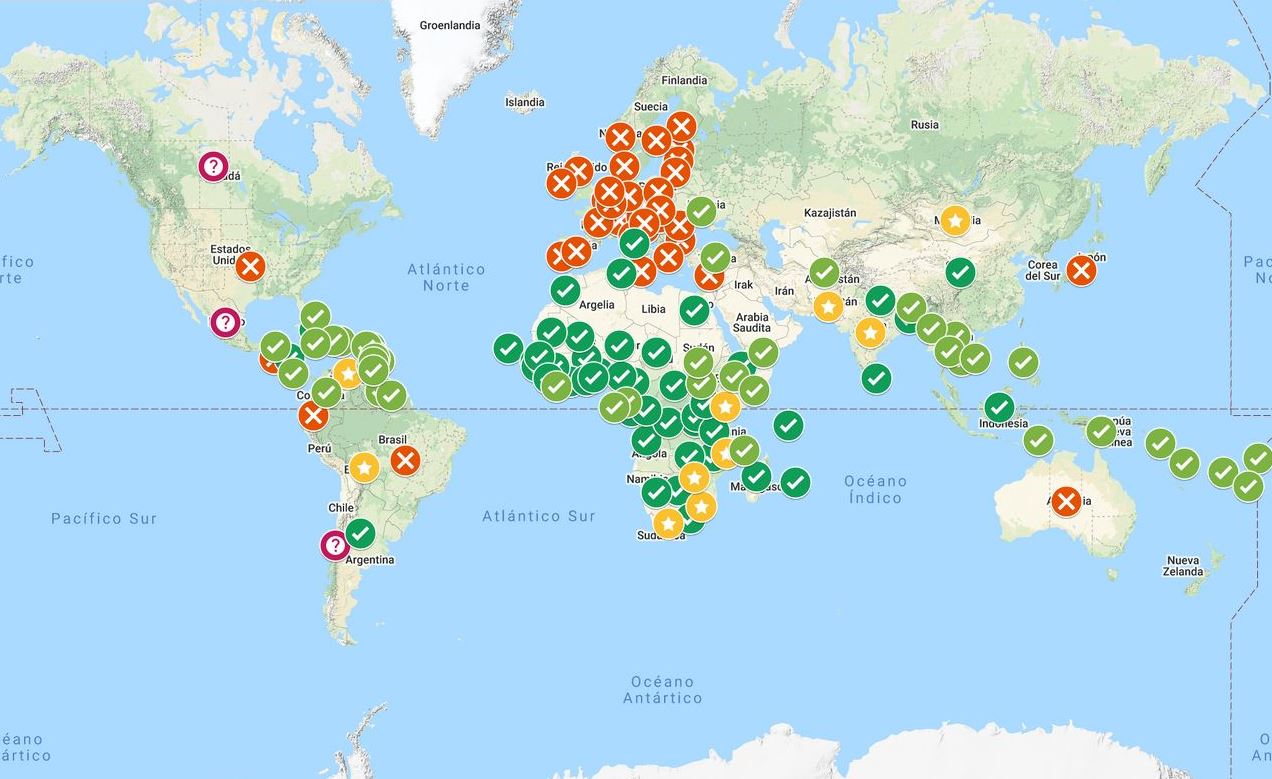
COVID-19 CONTINUED TO BE THE EPICENTRE OF ACCESS TO MEDICINES AND THE DEBATE ON THE R&D MODEL IN HEALTHCARE. COVID-19 VACCINES AND HEALTH TECHNOLOGY WERE STILL FAR FROM ACCESSIBLE FOR EVERYONE IN EVERY COUNTRY: WEALTHY COUNTRIES HOARDED AND JUST A TRICKLE REACHED COUNTRIES WITH FEWER RESOURCES. MEANWHILE, THE EUROPEAN UNION AND A SMALL NUMBER OF WEALTHY COUNTRIES CONTINUED TO BLOCK THE TEMPORARY SUSPENSION OF PATENTS AT THE WTO. THROUGHOUT THE YEAR WE WORKED WITH ORGANISATIONS ON A NATIONAL AND INTENATIONAL LEVEL TO SEARCH FOR CHANGE IN THIS EUROPEAN POSITION. IN ADDITION, WE ARE JOYFULLY CELEBRATING THE INCLUSION OF THE ELISA TEST IN C-TAP, DEVELOPED BY THE SPANISH NATIONAL RESEARCH COUNCIL (CSIC), SO IT COULD REACH MORE MANUFACTURERS AND COUNTIRES. SPAIN WAS THE FIRST COUNTRY TO REACH AN AGREEMENT OF THIS TYPE, DEMONSTRATING THAT THERE IS ANOTHER WAY OF DOING THINGS WITHIN R&D IN HEALTHCARE. THROUGH ITS NOT HEALTHY, WE CONTINUE OUR WORK TO UNCOVER THE ENORMOUS PUBLIC INVESTMENT IN VACCINE R&D; AND WE ARE IN COMMUNICATION WITH THE GOVERNMENT TO URGE THEM TO PROTECT RESEARCH IN ADVANCED TREATMENTS DEVELOPED IN OUR PUBLIC HOSPITALS.